Digital Therapeutics (DTx) (ASCII) from the viewpoint of patents --Yahoo! News
delivery
0 comments 0 commentsIn this series by ASCII, which collaborates with the Japan Patent Office on efforts to bring startups closer to intellectual property, and IP Tech Patent Business Corporation, which supports Tech companies with IP (intellectual property), Tech business players should know about intellectual property. We will deliver the points. [See more photos]
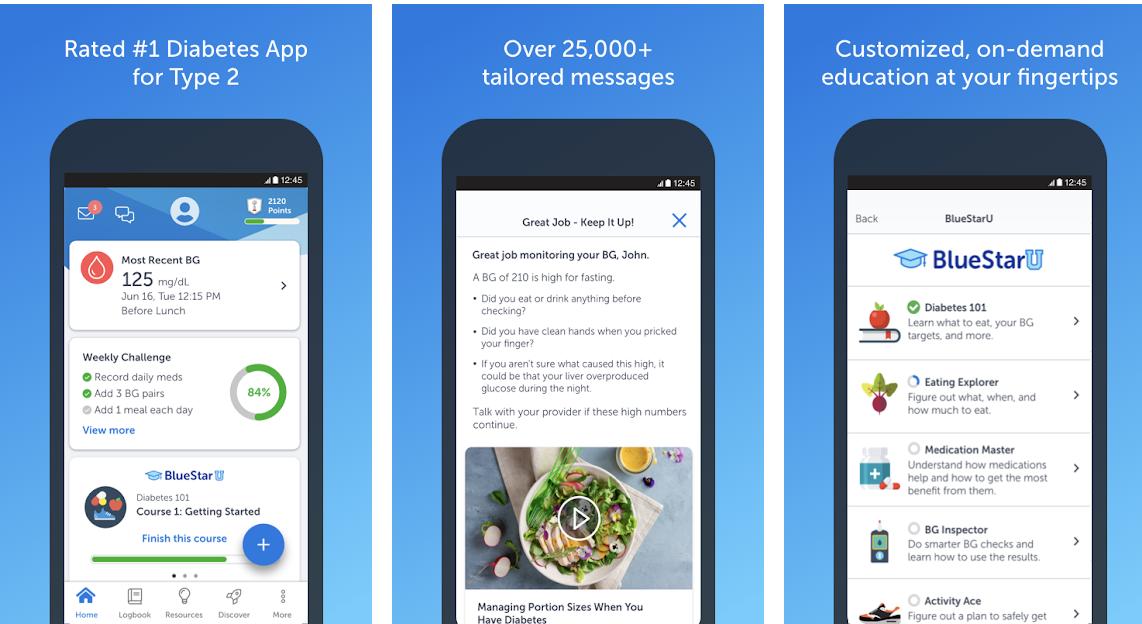
Photo: ASCII
In this series by ASCII, which collaborates with the Japan Patent Office on efforts to bring startups closer to intellectual property, and IP Tech Patent Business Corporation, which supports Tech companies with IP (intellectual property), Tech business players should know about intellectual property. We will deliver the points. In December 2020, CureApp's "CureApp SC" was covered by insurance in Japan. This is the first time that a therapeutic app has been made available for public health insurance and has received a lot of attention. In addition, CureApp applied for a regulatory application in May 2021 for the treatment of hypertension with the aim of subsequent approval and insurance coverage in 2022. Against this background, this paper will focus on digital therapeutics (DTx), which is expected to expand in the future as a countermeasure for lifestyle-related diseases and chronic diseases in Japan, where the population is aging. Table of Contents Digital Therapeutics: What is DTx? Check out famous DTx in Japan and overseas. Benefits obtained by combining DTx DTx and patents under development in Japan CureApp, Susmed, Save Medical IP Strategy Future patent strategy in DTx Future Digital Therapeutics: What is DTx? Actually, in Japan, what "DTx" stands for is not strictly defined. The computer program was added as a medical device after the revision of the Pharmaceutical Machinery Law in November 2014. What kind of program corresponds to the medical device program is described in the "Guidelines for Medical Device Applicability of the Program" formulated by the Ministry of Health, Labor and Welfare in March 2021. According to the guidelines, medical device programs have a purpose of use that falls under the definition of medical device, (1) by installing, etc., to give a general-purpose computer a function as a medical device, or (2) medical equipment that is tangible. It is described as being used in combination with the device. However, there is no definition for DTx in this guideline. DTx itself is clearly defined by the Digital Therapeutics Alliance (DTA), an industry group of digital treatment providers in the United States, which was established in 2017. According to DTA, DTx is defined as "evidence-based therapeutic intervention for the prevention, management and treatment of medical disorders and illnesses." It is different from digital health such as health promotion apps and wellness apps, which have begun to spread in recent years, in terms of "evidence-based therapeutic intervention". Products related to "digital health" in a broad sense do not have to meet the regulatory definition of medical devices, but products related to "DTx" support product claims such as risk, efficacy and purpose of use. In addition, it must be reviewed by a regulatory body and approved or certified as necessary. Check out the well-known DTx in Japan and overseas ・ BlueStar (Welldoc) The first application we will introduce is "BlueStar", an application that assists in the treatment of diabetic patients. It is the world's first DTx, and its therapeutic effect has been demonstrated in clinical trials in the United States, and it was approved by the US Food and Drug Administration (FDA) in 2010. BlueStar is installed on smartphones or tablets and is used by diabetics. When you enter your daily blood glucose level, the patient will be given a message that encourages a well-balanced diet and a message that encourages exercise based on the data. By using BlueStar in parallel with outpatient treatment, in addition to regular doctor guidance, continuous self-management is possible. Although it has not been commercialized in Japan yet, Astellas Pharma is aiming for commercialization in Japan from 2019. The clinical trial is scheduled to enter by March 2022. -AKL-T01 (US product name: EndeavorRx) (Akili) Next is the game-based application "AKL-T01" for attention deficit / hyperactivity disorder (ADHD) in children aged 8 to 12 years. The AKL-T01 was approved by the FDA in 2020, making it the world's first Böhm-based DTx. AKL-T01 is a game-style treatment that operates on smartphones and tablets and is designed to activate the prefrontal cortex of the brain, which is thought to play an important role in cognitive function. It monitors the progress of patients who have completed treatment sessions in seconds, continuously giving each patient the optimal level of task and helping them improve their performance. This is also not commercialized in Japan, but Shionogi is in the process of aiming for domestic commercialization from 2019. Currently, clinical trials are underway with the aim of obtaining regulatory approval. -CureApp SC Nicotine Addiction Treatment App and CO Checker (CureApp) This is the first product in Japan that has received regulatory approval and insurance coverage by CureApp mentioned above. It is a digital therapy that approaches the psychological dependence of nicotine addiction through an app and guides and treats the correct lifestyle by correctly changing the way of thinking and behavior of the patient. In June 2020, the product received the first regulatory approval in Japan as a therapeutic app. It was the first DTx in Asia and the world's first approval to assist in the treatment of nicotine addiction. Then, in November 2020, it was approved by the Central Social Insurance Medical Council (Chuikyo) of the Ministry of Health, Labor and Welfare as a target of public medical insurance. Even in January 2022, it will be the only app covered by insurance. CureApp SC nicotine addiction treatment app and CO checker are "patient app" that records the progress of treatment by the patient, "CO checker" that the patient measures the carbon monoxide concentration in the exhaled breath, and the doctor keeps track of the patient's situation. It consists of a "doctor app" that you can grasp. Patients enter their treatment status and carbon monoxide concentration as measured by the CO checker into the Patient app. The patient app then provides a personalized intervention. In addition, the doctor app allows doctors to see the daily progress gained from the patient app, allowing them to formulate appropriate treatment strategies for their patients. In May 2021, DTx CureApp, which is under development in Japan, applied for a regulatory application for a disease treatment program medical device as the second CureApp SC nicotine addiction treatment app and CO checker. We are aiming for regulatory approval and insurance coverage in 2022.・ Insomnia treatment app (Susmed) Susmed is developing an insomnia treatment app based on cognitive behavioral therapy. Treatment of insomnia in hospitals is generally prescribing sleeping pills. However, taking sleeping pills has side effects such as addiction, and it is said that it is difficult to lead to a fundamental treatment. An effective treatment is "cognitive-behavioral therapy," which changes the patient's lifestyle through doctor counseling and regular sleep records. The therapeutic app of SUSMED uses this "cognitive behavioral therapy". The validation trial for the regulatory approval application was completed in November 2021 with the result of achieving the primary endpoint. Currently, we are preparing to apply for regulatory approval. In addition, on December 27, 2021, it was announced that Shionogi Pharmaceutical has acquired the exclusive marketing rights for this app in Japan.・ Mobile application for diabetes management guidance (Save Medical) Save Medical is developing a therapeutic application that supports the treatment of diabetic patients. In this app, the patient himself inputs the lifestyle (diet, exercise, weight) and indicators (medication, blood pressure, blood sugar level), which are the basics of non-drug therapy for diabetes. Depending on the information entered, the app will automatically send a message to the patient to help with behavior change. Appropriate follow-up to individual patients improves behavioral indicators by increasing behavioral awareness of lifestyle-related disorders and poor medications that occur during the outpatient period and promoting behavior change. Save Medical started a clinical trial in August 2020 aimed at evaluating the efficacy and safety of this app for type 2 diabetes. We are aiming to obtain approval by the end of 2022. Benefits of the combination of DTx and patents In order to be DTx, it is necessary to obtain medical evidence from clinical trials and obtain regulatory approval as a medical device. And, in order to be approved for insurance coverage, it must be approved for public medical insurance by the Chuikyo of the Ministry of Health, Labor and Welfare. It is estimated that it will take about 5 to 6 years to develop a therapeutic application, conduct clinical trials, and obtain regulatory approval, and the cost will be about hundreds of millions to billions of yen. As you can see, it takes a long time and a lot of money to be approved as DTx. Currently, CureApp's CureApp SC is the only therapeutic app that has been approved in Japan, and there is no widespread knowledge of how to obtain regulatory approval. Therefore, if you can obtain regulatory approval, you can take an advantage over competitors even if you do not have a patent. However, if you have a patent, you can take a more advantageous position. For example, no other company can provide treatment support with a similar approach. In addition, due to the so-called patent linkage that considers the infringement of the patent right related to the original product in the approval procedure for the generic product, it is not possible to obtain regulatory approval by the same approach. At this time, latecomers need to develop treatments using different approaches, conduct separate clinical trials, and obtain regulatory approval, even if they are therapeutic apps to support the treatment of the same disease. On the other hand, if you do not have a patent, other companies can try to obtain regulatory approval with the same approach. It will take time and money, but other companies may be able to obtain regulatory approval. In this way, by obtaining a patent, it is possible to force other companies to develop apps from scratch, and it will be possible to extend the period in which they can be in an advantageous position. If a therapeutic app is approved as DTx, it will be possible to maintain a competitive advantage over other companies for a long time if it has a patent corresponding to the implementation of the therapeutic app. Below, we will look at the intellectual property of Japanese companies CureApp, Sasmed, and Save Medical. A company with a proven track record overseas is developing a therapeutic application in collaboration with a major domestic pharmaceutical company, but since overseas companies have already obtained patent rights in foreign countries, explanations are omitted here. To do. CureApp, Susmed, Save Medical IP Strategies-CureApp Patents for Smoking Cessation Patients: Patent 6116769, Patent 6339298 Patent 6116769 were filed on May 1, 2015 and March 31, 2017. It has been registered. This case has also been filed in China, Europe, Hong Kong, and the United States, and is patented in the United States. In addition, patent 6339298 was filed on April 20, 2017 and registered on May 18, 2018. This case has also been filed in China, Europe, and the United States, and is patented in the United States. Below, check the description of claim 1 respectively. Patent 6116769 [Claim 1] A program used for a non-smoking patient, in which information indicating the patient's current physical condition is input to a computer from a patient-side electronic device, which is an electronic device used by the patient, and a non-smoking medical treatment. At the stage of receiving patient interpretation information indicating the patient's interpretation of smoking-related matters input to the patient-side electronic device by the patient at the patient interpretation information acquisition timing determined based on at least one of the historical information. , The step of comparing the received patient interpretation information with the correct answer information about the smoking-related matters and determining whether or not the interpretation of the patient's smoking-related matters is incorrect. If it is determined that the patient's interpretation is incorrect, the patient will receive cognitive-behavioral therapy information containing information for correcting the incorrect interpretation of the patient's smoking-related matters based on the correct answer information. The stage of sending to an electronic device and the program that executes it. Patent 6339298 [Claim 1] A program used for non-smoking patients, in which the stage of receiving the declaration information indicating the presence or absence of smoking input by the patient and the concentration of the biometric index indicating the smoking state are displayed. The bioindex concentration measurement value based on the stage of receiving the bioindex concentration measurement value of the patient's biological sample measured by the bioindex concentration measuring device and the bioindex concentration measurement value and the bioindex concentration reference value. A stage of determining the consistency with the declared information, a stage of generating the smoking cessation treatment information for the smoking cessation treatment method to be executed for the patient based on the consistency judgment result, and the step of generating the above-mentioned generation. A program to send the smoking cessation treatment information to the patient's device and to execute it. In CureApp SC Nicotine Addiction Treatment App and CO Checker, the Patient App can be used for measurement results of exhaled carbon monoxide concentration measured using the CO Checker, smoking status entered by the patient, response to questions from the Patient App, etc. Based on this, we are trying to provide messages, videos, etc. that promote understanding of nicotine addiction and the establishment of behavioral changes related to smoking cessation. Patent 6116769 states that "providing messages, videos, etc. that promote understanding of nicotine addiction based on the response to questions from the patient app", and patent 6339298 states that "measured using a CO checker". Based on the measurement results of the exhaled carbon monoxide concentration and the smoking status entered by the patient, we will provide messages, videos, etc. that encourage the establishment of behavioral changes related to smoking cessation. " It can be seen that the features implemented in the CureApp SC Nicotine Addiction Treatment App and CO Checker are protected by Patent 6116769, Patent 6339298. CureApp also has a similar patent in the United States. Although the FDA has not yet approved it, there is also a US subsidiary, and we are proceeding with the regulatory application to the FDA in collaboration with the Japan office. By obtaining regulatory approval and patents in the United States as well, we are trying to advance our business in the United States as well. In addition, CureApp holds separate patents for programs for managing health-related information and programs for obese patients. In addition, the company received trademark registration for "Treatment App" on January 22, 2016, trademark registration for "Prescription App" on January 13, 2017, and multiple trademarks for "-Treatment App" in 2021. I have been registered. We can see the attitude of trying to establish an advantageous position by using trademarks as well. -Patent on Susmed's insomnia treatment support device: Patent 6245781, Patent 6928413 Patent 6245781 was filed on October 11, 2016 and registered on November 24, 2017. This case has also been filed in China, Europe, South Korea, Mexico, and the United States, and has been patented in South Korea and the United States. In addition, Patent 6928413 was filed on March 9, 2021 and registered on August 11, 2021. Check the description in claim 1 for each. Patent 6245781 [Claim 1] The sleep efficiency calculation unit that calculates the sleep efficiency of the user based on the information regarding the user's bedtime, sleep onset time, awakening time, and wake-up time, and the sleep efficiency calculation unit that calculates the sleep efficiency. Based on the sleep efficiency, the time setting unit that sets the bedtime of the user and the execution time of the drowsiness test first predetermined time before the bedtime, and the execution time set by the time setting unit or the second An insomnia treatment support device including a reminder message presenting unit that presents a message prompting the user to perform the sleepiness test when the time comes before or after a predetermined time. Patent 6928413 [Claim 1] Calculated by the sleep efficiency calculation unit that calculates the sleep efficiency of the user and the sleep efficiency calculation unit based on the information indicating the user's bedtime, sleep onset time, awakening time, and wake-up time. Based on the sleep efficiency, the user complies with the bedtime setting unit that sets the target bedtime and notifies the user, and the target bedtime set by the bedtime setting unit within a predetermined period. It is equipped with a compliance status index value calculation unit that calculates a compliance status index value indicating the status of whether or not it is present, and the sleep time setting unit is such that the sleep efficiency calculated by the sleep efficiency calculation unit is less than the first predetermined value. If there is, and the compliance status index value calculated by the compliance status index value calculation unit is equal to or greater than the threshold value, a time later than the previously set bedtime is set as the next bedtime, and the sleep efficiency calculation unit is used. When the sleep efficiency calculated by the above is less than the first predetermined value and the compliance status index value calculated by the compliance status index value calculation unit is less than the threshold value, it is the same as the previously set bedtime. Set the time to the next bedtime, and if the sleep efficiency calculated by the sleep efficiency calculation unit is equal to or greater than the second predetermined value, set the time earlier than the previously set bedtime to the next bedtime. A sleep onset treatment support device characterized by. By adjusting the bedtime based on "sleep efficiency", which represents the ratio of the time actually sleeping to the total time spent on the bed such as a bed, this promotes the behavior change of the patient and causes insomnia. It is an invention that will be resolved. Specifically, patent 6245781 sets the bedtime and drowsiness test implementation time based on sleep efficiency. In addition, Patent 6928413 sets the bedtime in more detail based on whether the set bedtime can be observed. It is not yet possible to clearly understand what functions have been found to be significant and safe in clinical trials, but considering the timing of filing and registration, it is considered that the contents of Patent 6928413 are included. Will be. From this, it can be expected that the function for which SUSMED is seeking regulatory approval is protected as a patent by patents 6245781 and 6928413. Also, although we do not yet have information on whether a regulatory application has been filed with the FDA, we may be aiming for a future application, referring to the fact that we have a patent in the United States. In addition to patents related to insomnia treatment support devices, SUSMED holds multiple patents for each business regarding the pillars of its business, "development of therapeutic apps" and "promotion of efficiency in clinical trials." doing. -Save Medical Inc. Information processing device that allows users to easily improve their lifestyle: Patent 6810496 Patent 6810496 was filed on July 27, 2020 and registered on December 15, 2020. The description of claim 1 is as follows. Patent 6810496 [Claim 1] An information processing device that presents a first task action, including a storage unit, an achievement level selection unit, and a task behavior setting unit. The storage unit includes behavior information and difficulty. The degree information and the category information are stored in association with each other, and the achievement degree selection unit is configured to be able to select an achievement option, a re-action option, or an unachieved option for the achievement degree of the first task action. When the unachieved option is selected in the achievement level selection unit, the action setting unit sets a second task action, which is less difficult than the first task action, with reference to the storage unit. An information processing device that sets a second task action of a different category with reference to the storage unit when the difficulty level of the first task action is the lowest. Since the first task behavior and the second task behavior are conceptual descriptions, check what each of them looks like from the description in the patent specification. According to paragraph [0016] of the patent specification, "the first task action 210 is, for example, insulin-independent diabetes, obesity, hyperlipidemia, hyperuricemia, cardiovascular disease, colon cancer, periodontal disease. For lifestyle-related diseases such as illness, squamous cell carcinoma of the lung, chronic bronchitis, pulmonary emphysema, and alcoholic liver disease, the task behavior may lead to improvement of lifestyle. The task behavior is diet and exercise. , Includes those related to smoking and drinking. " Further, according to paragraph [0017], "The second task behavior is a task behavior set next to the first task behavior 210 as an action that may lead to improvement of lifestyle for the above-mentioned lifestyle-related diseases. The task behaviors include those related to eating habits, those related to exercise habits, those related to rest, those related to smoking, and those related to drinking. " Patent 6810496 sets the user's lifestyle by setting an easier second task behavior when the first task behavior cannot be achieved, and setting a second task behavior in a different category when the first task behavior is the gentlest. I am trying to improve it easily. From the CureApp and Susmed patents, we were able to understand the functions implemented in the treatment app, but from the Save Medical patent, we feel that it is difficult to grasp the functions of the treatment app. Clinical trials must prove significance and safety. The therapeutic app that is being clinically tested may have more rigorous settings than the Task Behavior Setting Department described in Patent 6810496. Save Medical is likely to file an application for an invention that is in line with the implementation of a therapeutic app. Future of DTx's Patent Strategy It is currently uncertain how large the market will be for DTx. Under such circumstances, if the leading company obtains regulatory approval and obtains a patent, the second-ranked company will hesitate to compete. However, when it becomes clear that there is a large market and that DTx can be profitable, it is possible that competitors will emerge and patents will be obtained in anticipation of improvements to apps developed by predecessors. Currently, we are getting patents for inventions that are in line with the implementation of therapeutic apps, but in the future, we may be able to obtain multiple patents for improving therapeutic apps. Hmm. In addition, we can expect the market to grow for therapeutic apps for treating diseases with a large number of patients such as diabetes and hypertension, and we are responding to social demands such as reducing medical costs because drug prices can be suppressed. If it becomes clear that it is possible to obtain regulatory approval for an app that supports the treatment of diseases with a large number of patients, it is thought that major pharmaceutical companies will proceed with development, and it will be important to obtain patents. Can be done. In the pharmaceutical industry, one drug and one patent are common, and the value of patents is extremely high. On the other hand, in IT devices such as smartphones, one product often involves multiple patents, and the value and strength of patents are relatively weak compared to the pharmaceutical industry. In DTx, I think that one DTx may be protected by one patent, but it does not necessarily have to be protected one-on-one. Like the CureApp SC Nicotine Addiction Treatment App and CO Checker mentioned above, multiple patents can protect a single DTx. The value of patents in DTx is likely to be lower than in the pharmaceutical industry and higher than IT devices.About the author: IPTech Patent Business Corporation
Last updated: ASCII notebook-laptop
notebook-laptop